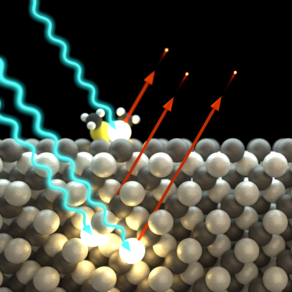
Photoelectron Spectroscopy (XPS/XPD)
X-ray photoelectron spectroscopy (XPS) enables an investigation of the chemical and electronic structure of samples with particular sensitivity to their surfaces and interfaces.
The method is based on the photoelectric effect, in which an incident photon is absorbed by an electron bound in the sample and this is emitted as a so-called photoelectron. By detecting and analyzing the energy of the photoelectrons, information is obtained about the chemical composition of a sample and the electronic structure of the atoms.
The method is particularly surface-sensitive because the photoelectrons, with their low kinetic energy, can only travel a short distance in the solid before being absorbed. The photoelectric equation describes the process quantitatively and links the kinetic energy to the element- and orbital-specific binding energy of the emitted photoelectrons.
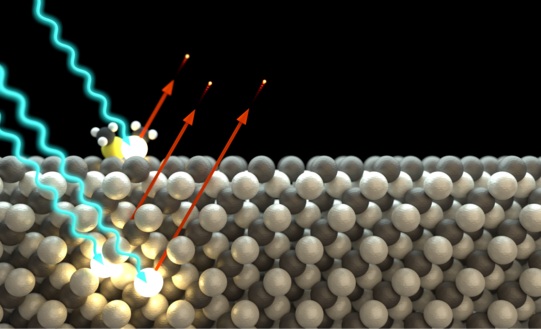
Using different excitation energies, different information of the sample can be obtained. For example, if one sample is irradiated with ultraviolet light (hv < 50 eV), the structure and state densities of the valence bands can be determined (UPS), while the use of X-rays (hv > 100 eV) provides information about the near-nuclear levels (XPS). These so-called hull levels each have binding energies characteristic of a chemical element, enabling the chemical analysis of a sample. Furthermore, small shifts in the binding energies also depend on the binding partner of an element (chemical shift), so that information about chemical bonds can be obtained from the analysis of the hull levels.
Photoelectron Diffraction (XPD)
Photoelectron diffraction is an extension of the XPS method for structure determination of surfaces and interfaces. It is based on the wave property of photoemitted electrons. The photoelectrons propagate through the solid as a matter wave and scatter elastically from neighboring atoms. The superposition of the scattered and unscattered electron waves results in a hemispherical diffraction pattern of modulated intensities, called anisotropy, which contains the complete structural information of the sample under investigation.
Such patterns can be recorded by measuring individual XPS spectra as a function of azimuth and polar angle and/or energy. The diffraction patterns are evaluated by comparison with simulated diffraction patterns, since a direct calculation of the structure from the measured intensity values is not possible due to the loss of the phase information of the electron wave functions.
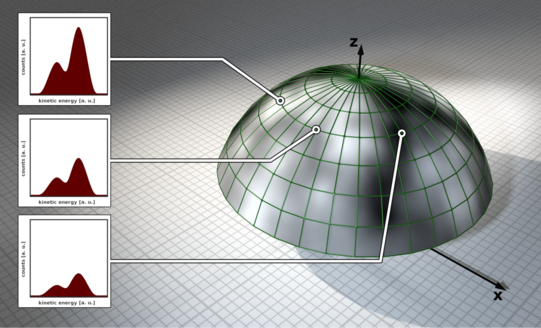
With the help of a simulation routine, a convergence of the calculated XPD patterns to the experimental diffractograms can be achieved by minimal variations of the structure, starting from an assumed starting structure. The agreement of the patterns is evaluated by means of the so-called R-factor. In this way, the atomic close order of a sufficiently complex structure can finally be resolved chemically selectively.